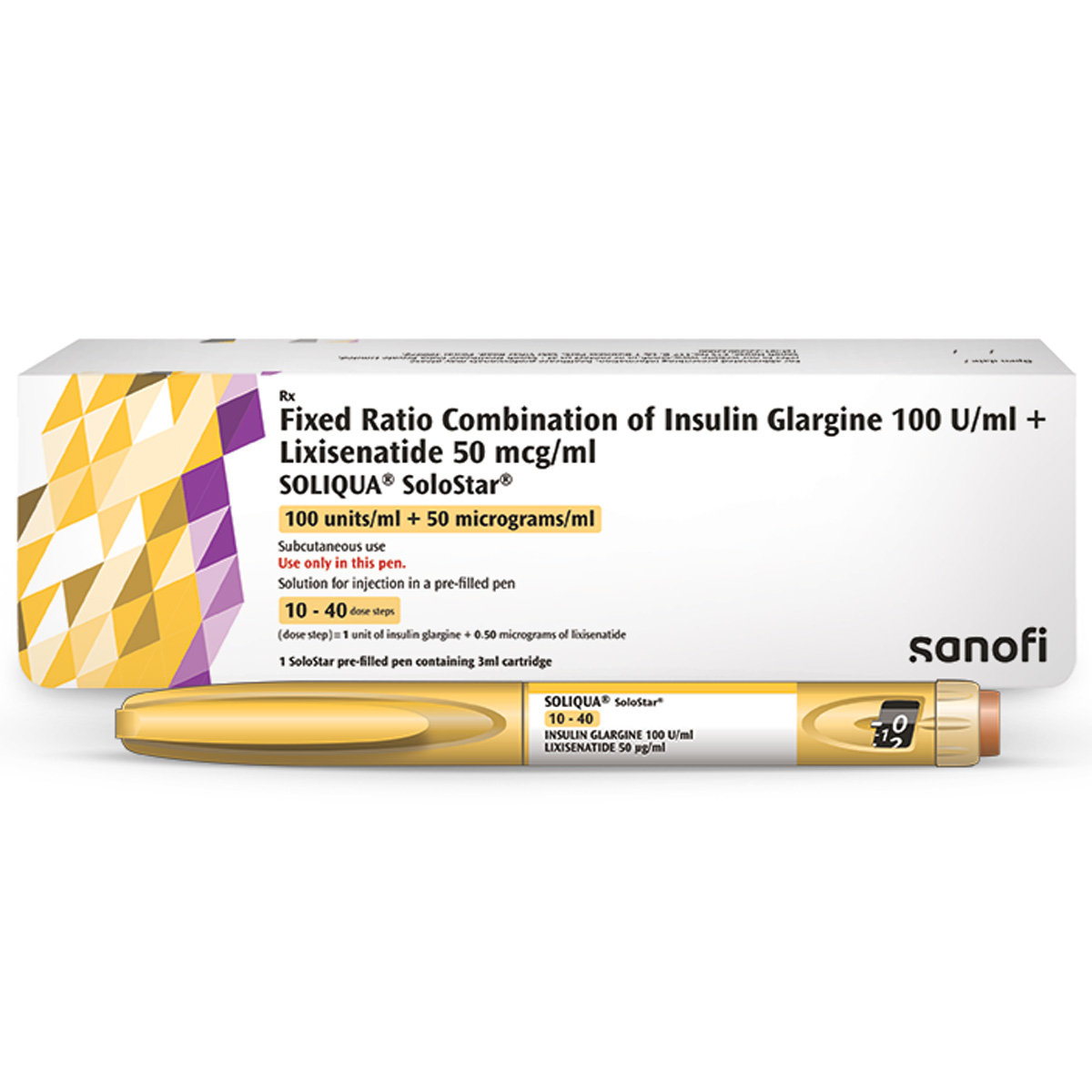
Soliqua Solostar Pre Filed Pen 3 ml

MRP ₹1850
(Inclusive of all Taxes)
₹277.5 Cashback (15%)
Soliqua Solostar Pre Filed Pen 3 ml is an Antidiabetic medicine. It is prescribed for those with high blood sugar, even though they consistently follow a balanced diet and exercise. It contains Insulin Glargine and Lixisenatide. Insulin Glargine is a long-acting insulin that provides a steady supply throughout the day, helping your body use glucose for energy. Lixisenatide stimulates insulin release after meals and reduces sugar production by the liver, leading to lower blood sugar levels.
Know Your Delivery Time
Provide Delivery Location

Secure Payment

India's Most Trusted Pharmacy

Genuine Products
Composition :
Manufacturer/Marketer :
Consume Type :
Return Policy :
Expires on or after :
About Soliqua Solostar Pre Filed Pen 3 ml
Soliqua Solostar Pre Filed Pen 3 ml is an Antidiabetics medicine. It is recommended for adults with type 2 diabetes mellitus as a supplement to diet and exercise to improve glycemic control. Type 2 diabetes (T2DM) is a chronic (long-term) disorder that affects the body's ability to regulate blood sugar. Insulin is a hormone that regulates how the body uses glucose for energy. Type 2 diabetes occurs when the body fails to produce enough insulin or does not use it adequately.
Soliqua Solostar Pre Filed Pen 3 ml contains Insulin Glargine and Lixisenatide. Insulin Glargine is a long-acting insulin that provides a steady supply throughout the day, helping your body use glucose for energy. Lixisenatide is a glucagon-like peptide-1 (GLP-1) receptor agonist, stimulating insulin release after meals and reducing sugar production by the liver, leading to lower blood sugar levels.
Sometimes, you may experience injection site reactions, such as lumps under the skin. The injection site should be rotated to prevent skin changes. You may also experience hypoglycaemia (symptoms include sweating, clammy skin, anxiety, fast heartbeat), dizziness, nausea, diarrhoea, and vomiting. Most of these side effects of Soliqua Solostar Pre Filed Pen 3 ml do not require medical attention and gradually resolve over time. However, if the side effects persist, contact your doctor.
Try not to stop taking Soliqua Solostar Pre Filed Pen 3 ml of your own. Let your doctor know about this, as it may cause withdrawal symptoms. Do not take Soliqua Solostar Pre Filed Pen 3 ml if you have any low blood glucose levels, kidney, liver, or heart problems, or problems with alcohol or other prescription recreational drugs. Along with Soliqua Solostar Pre Filed Pen 3 ml, you should take a healthy diet, exercise regularly, and maintain weight as your doctor advises. Soliqua Solostar Pre Filed Pen 3 ml is a cold chain medicine, so it must be stored in the refrigerator between 2-8 degrees Celsius; otherwise, its efficiency might get lost. Do not store it in the fridge freezer.
Uses of Soliqua Solostar Pre Filed Pen 3 ml
Soliqua Solostar Pre Filed Pen 3 ml is used in the treatment of Type 2 diabetes mellitus. The detailed use of Soliqua Solostar Pre Filed Pen 3 ml is as follows:
- Management of type 2 diabetes mellitus: Soliqua Solostar Pre Filed Pen 3 ml is used to manage type 2 diabetes mellitus.

Have a query?
Directions for Use
- Soliqua Solostar Pre Filed Pen 3 ml should be injected 1 hour before the first meal of the day.
- It is recommended to administer one injection once daily at the same time each day or as advised by your doctor.
- Check the label for instructions before using Soliqua Solostar Pre Filed Pen 3 ml.
- It should be administered under the skin (subcutaneously). It must not be given intravenously, intramuscularly, or by an infusion pump.
- To use an insulin pen, remove the cap, attach a new needle, and set the correct dose. Inject the needle, then slowly push the injection button to deliver the full dose. Hold the pen for 10 seconds before pulling it out.
- Change injection sites within the same area (abdomen, thigh, or upper arm) to prevent the formation of hard lumps.
- If you are not well-trained to self-administer Soliqua Solostar Pre Filed Pen 3 ml, ask a healthcare professional to administer it.
Medicinal Benefits
Soliqua Solostar Pre Filed Pen 3 ml is a long-acting insulin that provides a steady supply throughout the day, helping your body use glucose for energy. It also helps improve glycemic control. By effectively managing blood sugar, Insulin Glargine-Lixisenatide can help reduce the risk of long-term complications associated with type 2 diabetes, such as heart disease, stroke, kidney problems, and nerve damage.
How Soliqua Solostar Pre Filed Pen 3 ml Works
Storage
What if I have taken an overdose of Soliqua Solostar Pre Filed Pen 3 ml
Drug Warnings
Before taking the Soliqua Solostar Pre Filed Pen 3 ml, inform your doctor about all your medical conditions (especially if you have hypoglycaemia), sensitivities (especially allergic to any component present in Soliqua Solostar Pre Filed Pen 3 ml), and all medications you are using to rule out any side effects. If you are pregnant or breastfeeding, do not consume Soliqua Solostar Pre Filed Pen 3 ml unless recommended by the doctor. Soliqua Solostar Pre Filed Pen 3 ml causes drowsiness and dizziness, so drive only if you are alert. Soliqua Solostar Pre Filed Pen 3 ml is not recommended for children as the safety and efficacy have not been established. Soliqua Solostar Pre Filed Pen 3 ml should be taken with caution, especially if you have a history of liver or kidney impairment. Frequent glucose monitoring and dose adjustment may be necessary for patients with liver or kidney impairment. Care should be taken while travelling across more than two time zones. Your doctor may adjust your insulin schedule.
Diet & Lifestyle Advise
Essential diet and lifestyle changes that can significantly benefit type 2 diabetes (T2D) patients:
Diet:
- Focus on whole, unprocessed foods: Prioritize fruits, vegetables, whole grains (brown rice, quinoa, oats), and lean proteins (fish, chicken, beans). These foods provide sustained energy, regulate blood sugar, and are rich in essential nutrients.
- Limit processed carbohydrates and sugary drinks: Sugary drinks, white bread, pastries, and refined carbohydrates can cause blood sugar spikes. Opt for whole-wheat versions or healthier alternatives like water and unsweetened tea/coffee.
- Fiber is your friend. Fibre slows down sugar absorption, promoting healthy blood sugar levels. For a good fibre intake, include plenty of fruits, vegetables, and whole grains.
- Healthy fats are essential: Unsaturated fats from sources like nuts, seeds, and olive oil can aid in satiety and improve heart health, which is crucial for diabetics.
- Portion control is vital: Be mindful of portion sizes to avoid overeating and manage calorie intake.
Lifestyle:
- Regular exercise is essential: Aim for at least 120-150 minutes of moderate-intensity exercise (swimming, brisk walking) or 75 minutes of vigorous exercise (running, cycling) spread throughout the week. Physical activity helps your body use insulin more effectively and lowers blood sugar levels.
- Regular sleep is crucial: Aim for 7-8 hours of quality sleep each night. Poor sleep can disrupt hormones that regulate blood sugar and appetite.
- Eat meals: Skipping meals can disrupt your blood sugar levels. Consistency in meal timing is essential.
- Maintain a healthy weight: If you're obese or overweight, losing even a moderate amount of weight can significantly improve blood sugar control.
- Stay hydrated: Drinking plenty of water helps your body function optimally and regulate blood sugar levels.
- Manage stress: Chronic stress can elevate blood sugar levels. Explore relaxation techniques like yoga, meditation, or deep breathing to manage stress effectively.
- Regular doctor visits: Schedule regular checkups with your doctor to monitor your blood sugar, discuss your management plan, and identify any potential complications.
Habit Forming
Therapeutic Class
Alcohol
Unsafe
You are recommended not to consume alcohol along with Soliqua Solostar Pre Filed Pen 3 ml to avoid unpleasant side effects. Alcohol may either decrease or increase the blood sugar level, which can be fatal.
Pregnancy
Caution
The potential risk for humans is unknown. Soliqua Solostar Pre Filed Pen 3 ml should not be used during pregnancy. If a patient wishes to become pregnant or pregnancy occurs, treatment with Soliqua Solostar Pre Filed Pen 3 ml should be discontinued.
Breast Feeding
Caution
There are no adequate studies in women for determining infant risk when using this medication during breastfeeding. Inform your doctor. Your doctor will weigh the potential benefits against the potential risks before taking this medication while breastfeeding.
Driving
Caution
Drive with caution; Soliqua Solostar Pre Filed Pen 3 ml usually causes drowsiness and affects driving ability. Your ability to concentrate and react may be reduced if you have hypoglycaemia (low blood sugar).
Liver
Caution
If you have liver disease inform your doctor before taking Soliqua Solostar Pre Filed Pen 3 ml. Frequent glucose monitoring and dose adjustment may be necessary for patients with liver impairment.
Kidney
Caution
Soliqua Solostar Pre Filed Pen 3 ml is not recommended in patients with end-stage renal disease. Frequent glucose monitoring and dose adjustment may be necessary for patients with renal impairment.
Children
Caution
Safety and efficacy have not been established.
Heart
Soliqua Solostar Pre Filed Pen 3 ml may be used in heart patients but requires caution due to potential effects on heart rate and gastrointestinal symptoms. Always consult your doctor before starting to ensure it's safe for your condition.
Geriatrics
Caution
Soliqua Solostar Pre Filed Pen 3 ml can be used in geriatric patients, but with careful monitoring due to age-related risks like kidney issues or gastrointestinal side effects. Individualized treatment and regular follow-up are essential to ensure safety and effectiveness.
FAQs
Soliqua Solostar Pre Filed Pen 3 ml is Indicated as an adjunct to diet and exercise to improve glycaemic (sugar) control in adults with type 2 diabetes mellitus
1. Improved blood sugar control. 2. Reduced risk of long-term complications from diabetes. 3. Once-daily injection for simplified treatment. 4. There is a lower risk of hypoglycaemia (low blood sugar) compared to other medications.
Soliqua Solostar Pre Filed Pen 3 ml might interact with other medications, especially other diabetes medications. It's crucial to tell your doctor about all medications you take before starting Soliqua Solostar Pre Filed Pen 3 ml.
Soliqua Solostar Pre Filed Pen 3 ml is a cold-chain medicine that must be stored at 2- 8 degrees Celsius; otherwise, its efficiency in reducing blood glucose levels declines. Do not keep it inside the freezer.
Country of origin
Manufacturer/Marketer address
Customers Also Bought
Disclaimer
Keep Refrigerated. Do not freeze.
Author Details
We provide you with authentic, trustworthy and relevant information
Reference
- https://reference.medscape.com/drug/soliqua-100-33-insulin-glargine-lixisenatide-1000084
- https://www.sanofi.in/dam/jcr:f13d905d-adc5-4f0e-aa8c-e2f0c3a711a1/Soliqua%C2%AE%20SoloStar%C2%AE%20PI.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208673s002lbl.pdf
- https://medlineplus.gov/druginfo/meds/a600027.html#:~:text=Insulin%20glargine%20is%20a%20long,liver%20from%20producing%20more%20sugar.
- https://www.drugs.com/drug-interactions/insulin-glargine-lixisenatide-index.html?filter=3
Buy best Diabetics products by
Torrent Pharmaceuticals Ltd
Intas Pharmaceuticals Ltd
Eris Life Sciences Ltd
Sun Pharmaceutical Industries Ltd
Lupin Ltd
Micro Labs Ltd
Mankind Pharma Pvt Ltd
Cipla Ltd
Lloyd Healthcare Pvt Ltd
Macleods Pharmaceuticals Ltd
Abbott India Ltd
Alkem Laboratories Ltd
Primus Remedies Pvt Ltd
Glenmark Pharmaceuticals Ltd
Aristo Pharmaceuticals Pvt Ltd
Dr Reddy's Laboratories Ltd
Wockhardt Ltd
USV Pvt Ltd
La Renon Healthcare Pvt Ltd
Emcure Pharmaceuticals Ltd
Ajanta Pharma Ltd
Ipca Laboratories Ltd
Alembic Pharmaceuticals Ltd
Fusion Health Care Pvt Ltd
Corona Remedies Pvt Ltd
Hbc Life Sciences Pvt Ltd
East West Pharma India Pvt Ltd
Eswar Therapeutics Pvt Ltd
Elbrit Life Sciences Pvt Ltd
Alteus Biogenics Pvt Ltd
Medley Pharmaceuticals Ltd
Zydus Healthcare Ltd
Ranmarc Labs
Sinsan Pharmaceuticals Pvt Ltd
Mitoch Pharma Pvt Ltd
Msn Laboratories Pvt Ltd
Akumentis Healthcare Ltd
Arkas Pharma Pvt Ltd
Unison Pharmaceuticals Pvt Ltd
Acmedix Pharma Llp
Sanofi India Ltd
Novo Nordisk India Pvt Ltd
Tas Med India Pvt Ltd
Leeford Healthcare Ltd
Q Check Pharmaceuticals
Blue Cross Laboratories Pvt Ltd
Xemex Life Sciences
Anthem Bio Pharma
Sydmen Life Sciences Pvt Ltd
Aareen Healthcare Pvt Ltd
Diacardus Pharmacy Pvt Ltd
Jubilant Lifesciences Ltd
Neucure Lifesciences Pvt Ltd
Nirvana India Pvt Ltd
Stature Life Sciences Pvt Ltd
Systopic Laboratories Pvt Ltd
Talent India Pvt Ltd
Alvio Pharmaceuticals Pvt Ltd
Panacea Biotec Ltd
Shrrishti Health Care Products Pvt Ltd
Spectra Therapeutics Pvt Ltd
Edoc Life Sciences Pvt Ltd
Franco Indian Pharmaceuticals Pvt Ltd
Akesiss Pharma Pvt Ltd
Elinor Pharmaceuticals (P) Ltd
Hicxica Formulations Pvt Ltd
Indoco Remedies Ltd
Saan Labs
Zydus Cadila
Biocon Ltd
Eli Lilly and Company (India) Pvt Ltd
Remedy Life Sciences Pvt Ltd
Verse Lifesciences
Capital Pharma
Koye Pharmaceuticals Pvt Ltd
Sanz Pharmaceuticals
Lippon Pharma Pvt Ltd
MEDICAMEN BIOTECH LTD
Morepen Laboratories Ltd
Atos Lifesciences Pvt Ltd
Azkka Pharmaceuticals Pvt Ltd
Converge Biotech Pvt Ltd
Erinyle Health Care Pvt Ltd
MERAKI HEALTH
FDC Ltd
Jarun Pharmaceuticals Pvt Ltd
Knoll Healthcare Pvt Ltd
Opsis Care Lifesciences Pvt Ltd
Vasu Organics Pvt Ltd
Daylon healthcare pvt Ltd
Elder Pharmaceuticals Ltd
Orris Pharmaceuticals
Cadomed Pharmaceuticals India Pvt Ltd
Erinyle Pharma
Medicure Life Sciences Pvt Ltd
N Line Healthcare Pvt Ltd
Olcare Laboratories Pvt Ltd
RPG Life Sciences Ltd
Wallace Pharmaceuticals Pvt Ltd
Zuventus Healthcare Ltd