CTD-M 6.25/50 Tablet 10's
MRP ₹141.5
(Inclusive of all Taxes)
₹21.2 Cashback (15%)
Know Your Delivery Time
Provide Delivery Location

Secure Payment

India's Most Trusted Pharmacy

Genuine Products
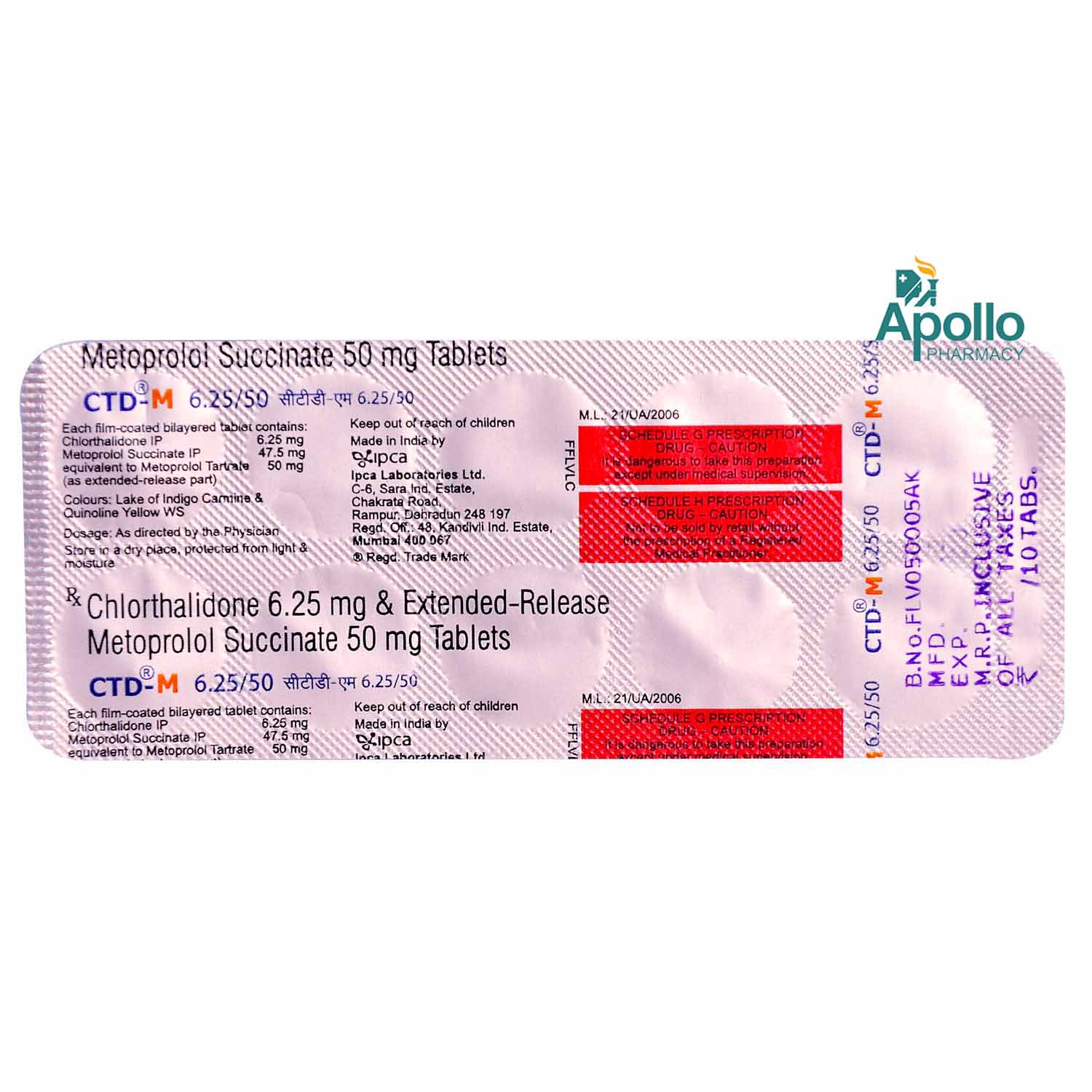
Composition :
Manufacturer/Marketer :
Consume Type :
Return Policy :
Expires on or after :
About CTD-M 6.25/50 Tablet
CTD-M 6.25/50 Tablet is used to treat hypertension (high blood pressure). It can also reduce oedema (fluid retention) in hypertensive patients. Hypertension is a condition in which there will be a persistent rise in blood pressure levels. This condition can also cause fluid retention, leading to peripheral oedema (swelling of legs and hands).
CTD-M 6.25/50 Tablet is a combination of two medicines: Metoprolol and Chlorthalidone. Metoprolol is a beta-blocker and works by blocking epinephrine, resulting in a decrease in heart rate and blood pressure. Chlorthalidone is a diuretic or water pill that acts by expelling excess water and salt from the body. It helps in decreasing fluid accumulation and oedema.
You should take this medicine as prescribed by your doctor. CTD-M 6.25/50 Tablet may cause some side effects such as anorexia (loss of appetite), nausea and vomiting, diarrhoea, constipation, jaundice (yellowing of the whites of eyes and skin), dizziness, headache, visual abnormality, photosensitivity (sensitivity to light), orthostatic hypotension (dizziness on standing due to low blood pressure), weakness, restlessness, bradycardia (slow heart rate), and stomach pain. It may also electrolyte levels. If any of these side effects persist, consult your doctor immediately.
Do not take CTD-M 6.25/50 Tablet if you are allergic to any contents of it. Do not take CTD-M 6.25/50 Tablet if you have heart rhythm problems, heart failure, blocked blood vessels, bradycardia (slow heart rate), chest pain, metabolic acidosis (increased acidity in the blood), and hypotension (low blood pressure). CTD-M 6.25/50 Tablet should be used with caution in patients with kidney or liver diseases, allergy or asthma, and gout (increased uric acid levels in the blood). Also, inform your doctor if you are pregnant and breastfeeding.
Uses of CTD-M 6.25/50 Tablet
CTD-M 6.25/50 Tablet is used in the treatment of Hypertension (high blood pressure), and edema (fluid overload). The detailed uses of CTD-M 6.25/50 Tablet are as follows:
- Treatment: CTD-M 6.25/50 Tablet helps to treat edema by reducing excess fluid accumulation in the body’s tissues, often associated with conditions like heart failure, liver cirrhosis, or kidney disorders.
- Hypertension Management: CTD-M 6.25/50 Tablet is used as an adjunct therapy to help manage hypertension by lowering blood pressure.
- Congestive Heart Failure: CTD-M 6.25/50 Tablet helps to manage congestive heart failure by reducing fluid retention, alleviating symptoms, and improving quality of life and physical activity for patients.
- Improvement in heart and kidney function: CTD-M 6.25/50 Tablet helps to improve heart and kidney function by reducing excess fluid in the body, preventing complications like swelling, shortness of breath, and strain on the heart and kidneys.

Have a query?
Directions for Use
- Recommended to take CTD-M 6.25/50 Tablet with food or as advised by a doctor.
- Follow your doctor's instructions on the dosage and timing of this medication, to ensure the safety.
- Swallow the medicine as a whole with a glass of water.
- Do not crush, break, or chew it.
Medicinal Benefits
CTD-M 6.25/50 Tablet is a combination of two medicines: Metoprolol and Chlorthalidone. Metoprolol is a beta-blocker and works by acting on epinephrine, a hormone that can increase heart rate and blood pressure. So, blocking the action of epinephrine can result in a decrease in heart rate and blood pressure. Chlorthalidone is a diuretic or water pill that acts by expelling excess water and salt from the body. It helps in decreasing fluid accumulation and swelling or edema.
How CTD-M 6.25/50 Tablet Works
Storage
- If you experience low blood pressure symptoms like dizziness, lightheadedness, or fainting while taking medication, seek immediate medical attention.
- Make lifestyle modifications and adjust your medication regimen under medical guidance to manage low blood pressure.
- As your doctor advises, regularly check your blood pressure at home. Record your readings to detect any changes and share them with your doctor.
- Fluid intake plays a vital role in managing blood pressure by maintaining blood volume, regulating blood pressure, and supporting blood vessel function. Drinking enough fluids helps prevent dehydration, maintain electrolyte balance, and regulate fluid balance.
- Take regular breaks to sit or lie down if you need to stand for long periods.
- When lying down, elevate your head with extra pillows to help improve blood flow.
- Avoid heavy exercise or strenuous activities that can worsen low blood pressure.
- Wear compression socks as your doctor advises to enhance blood flow, reduce oedema, and control blood pressure.
- If symptoms persist or worsen, or if you have concerns about your condition, seek medical attention for personalized guidance and care.
- Exercising regularly helps lower the risk of heart problems.
- Maintain a healthy diet, including vegetables and fruits.
- Rest well; get enough sleep.
- Manage stress with yoga and meditation.
- Limit alcohol and smoking.
- Talk to your doctor about oral potassium supplements.
- Eat potassium rich foods such as bananas, avocados, oranges, dark leafy greens, beans and peas, fish, spinach, milk and tomatoes.
- Limit intake of full-fat dairy products, red meat and processed foods rich in saturated fats.
- Avoid foods with label “partially hydrogenated oils”.
- Eat more vegetables, fruits, oats, whole grains, lentils and beans to increase fiber intake.
- Include foods rich in monounsaturated fats like nuts, avocados, olive oil, and oily fish.
- Limit consumption of egg yolks.
- Exercise for at least 30 minutes 5 days a week.
- Aim for weight loss and maintain healthy weight.
- Quit smoking as smoking raises the risk of heart disease associated with cholesterol.
- Limit alcohol consumption as excessive drinking may raise cholesterol levels.
- Reducing the amount of time you spend outside and indoors in the cold.
- keeping your hands warm by donning mittens, gloves, or other protective clothing.
- Observing a skincare regimen that safeguards your fingers and hands.
- To improve circulation, give your hands and feet a little massage.
- Drink black or green tea for antioxidants.
- Eat foods rich in omega- 3 adipose acids.
- Eat iron-rich foods or take iron supplements if anemic.
- Consume berries, beets, onions, bananas, and dark leafy flora.
- Add shiitake mushrooms for vascular health.
- Eat yogurt for calcium, magnesium, and potassium.
- Exercise regularly.
- Avoid smoking.
- Drop stress.
- Consider drinking gusto tea as a natural vasodilator.
What if I have taken an overdose of CTD-M 6.25/50 Tablet
Drug Warnings
CTD-M 6.25/50 Tablet may cause abnormalities such as electrolyte imbalances such as a decrease in potassium, sodium or chloride levels. So, it is advised to monitor electrolyte levels before initiating this therapy and also should be used with extreme caution in patients with severe liver and kidney disease. CTD-M 6.25/50 Tablet may cause photosensitivity, so avoid exposure to sunlight for prolonged periods. CTD-M 6.25/50 Tablet contains lactose, so inform your doctor if you have any intolerance to sugars.
Drug-Drug Interactions
Drug-Drug Interactions
Login/Sign Up
Co-administration of CTD-M 6.25/50 Tablet and cisapride may increase the risk or severity of an irregular heart rhythm that may be serious.
How to manage the interaction:
Taking CTD-M 6.25/50 Tablet with Cisapride is not recommended, please consult your doctor before taking it. Do not discontinue the medication without consulting a doctor.
Co-administration of Aminolevulinic acid and CTD-M 6.25/50 Tablet may increase the risk of severe sunburn.
How to manage the interaction:
Taking CTD-M 6.25/50 Tablet with Aminolevulinic acid together can possibly result in an interaction, but it can be taken if a doctor has advised it. If you notice that your skin is very sensitive to sunlight or if you have a really bad sunburn, it's important to contact your doctor right away. Do not discontinue any medications without consulting a doctor.
Taking Droperidol can make CTD-M 6.25/50 Tablet less effective in lowering blood pressure.
How to manage the interaction:
Taking CTD-M 6.25/50 Tablet with Droperidol together can possibly result in an interaction, but it can be taken if your doctor has advised it. If you have any of these symptoms, it's important to contact your doctor right away. These symptoms include an irregular heart rhythm, severe or long-lasting diarrhea or vomiting, complications, sudden dizziness or lightheadedness, fainting, difficulty breathing, heart palpitations, weakness, feeling sleepy or confused, muscle pain, and feeling nauseous or vomiting. Do not stop using any medications without first talking to your doctor.
Taking CTD-M 6.25/50 Tablet can make the body eliminate Arsenic trioxide faster, leading to lower levels in the blood and potentially reducing its effectiveness.
How to manage the interaction:
Co-administration of CTD-M 6.25/50 Tablet with Arsenic trioxide can possibly result in an interaction, but it can be taken if a doctor has advised it. If you have any of these symptoms, it's important to contact a doctor right away: your heart beating irregularly, feeling dizzy or lightheaded, fainting, having a fast or pounding heartbeat, feeling weak or drowsy, being confused, experiencing muscle pain, or feeling nauseous or vomiting. Do not discontinue any medications without consulting a doctor.
Co-administration of Tizanidine and CTD-M 6.25/50 Tablet may show additive effects and increase the risk of low blood pressure.
How to manage the interaction:
Although there is an interaction, CTD-M 6.25/50 Tablet can be taken with tizanidine if prescribed by the doctor. If you experience headache, dizziness, palpitations, or sweating, contact your doctor immediately. Do not discontinue any medications without consulting a doctor.
Co-administration of CTD-M 6.25/50 Tablet with pimozide can increase the risk of an irregular heart rhythm that may be serious.
How to manage the interaction:
Although there is an interaction, CTD-M 6.25/50 Tablet can be taken with pimozide if prescribed by the doctor. Consult the prescriber if you develop sudden dizziness, lightheadedness, fainting, or fast or pounding heartbeats during treatment with pimozide. Also, let the doctor know if you experience signs of electrolyte disturbance, such as weakness, tiredness, drowsiness, confusion, muscle pain, cramps, dizziness, nausea, or vomiting. Do not discontinue any medications without consulting a doctor.
Co-administration of CTD-M 6.25/50 Tablet together with lithium can increase the effects of lithium.
How to manage the interaction:
Although there is a interaction between CTD-M 6.25/50 Tablet and lithium, but it can be taken together if prescribed by a doctor. However, consult your doctor if you experience diarrhea, vomiting, drowsiness, shaking of hands and legs, thirst, increased urination, lack of coordination, or muscle weakness. Do not discontinue any medications without consulting a doctor.
Co-administration of CTD-M 6.25/50 Tablet with ziprasidone can increase the risk of an irregular heart rhythm that may be serious.
How to manage the interaction:
Although there is an interaction, CTD-M 6.25/50 Tablet can be taken with ziprasidone if prescribed by the doctor. but it can be taken together if prescribed by a doctor. However, consult your doctor if you experience sudden dizziness, lightheadedness, fainting, shortness of breath, weakness, tiredness, drowsiness, confusion, muscle pain, cramps, nausea, or vomiting. Do not discontinue any medications without consulting a doctor.
Coadministration of CTD-M 6.25/50 Tablet with amiodarone may increase the risk of abnormal heart rhythms.
How to manage the interaction:
Although taking CTD-M 6.25/50 Tablet with amiodarone may cause an interaction, it is safe to do so if your doctor has prescribed it. If you have a cardiac problem or electrolyte imbalances, you may be more vulnerable. If you develop sudden dizziness, lightheadedness, fainting, shortness of breath, or a rapid heartbeat, consult a doctor. Do not stop taking any medications without visiting a doctor.
Coadministration of Acebutolol with CTD-M 6.25/50 Tablet can cause abnormal heart rhythm.
How to manage the interaction:
Taking CTD-M 6.25/50 Tablet and Acebutolol together can possibly result in an interaction, it can be taken if your doctor has advised it. However, if you experience blurry vision, confusion, dizziness, fainting, lightheadedness, nausea or vomiting, contact your doctor immediately. Do not discontinue any medications without consulting a doctor.
Drug-Food Interactions
Drug-Food Interactions
Login/Sign Up
Diet & Lifestyle Advise
- Keep your weight under control with BMI (Body Mass Index) 19.5-24.9.
- Do regular physical activity or exercise for at least 150 minutes per week, or about 30 minutes most days of the week. Doing this can help you to lower your raised blood pressure by about 5 mm of Hg.
- Opt for a diet rich in whole grains, fruits, veggies, and low-fat dairy products.
- If you are taking alcohol then only one serving for women and two servings for men is advisable.
- Quitting smoking is the best strategy to lower the risk of heart disease.
- Avoid chronic stress as it can raise your blood pressure. Try to enjoy and spent time with your loved ones to cope with stress and practice mindfulness techniques.
- Monitor your blood pressure daily and if there is too much fluctuation then immediately contact your doctor.
- Try to include heart-healthy omega 3 fatty acids containing food drinks in your daily diet. You can also use low-fat cooking oil like olive oil, soybean oil, canola oil, and coconut oil can help in lowering your elevated blood pressure.
Habit Forming
Therapeutic Class
All Substitutes & Brand Comparisons
RX
Out of StockCLOVET M 50MG TABLET
Ajanta Pharma Ltd
₹69
(₹6.21 per unit)
51% CHEAPERRX
Revelol CH 50/6.25 Tablet 10's
Ipca Laboratories Ltd
₹141.5
(₹12.74 per unit)
Alcohol
Unsafe
CTD-M 6.25/50 Tablet may interact with alcohol and worsen the condition.
Pregnancy
Consult your doctor
CTD-M 6.25/50 Tablet is a category C drug. CTD-M 6.25/50 Tablet should not be used in pregnant women unless clinically required and when benefits outweigh the risks.
Breast Feeding
Consult your doctor
CTD-M 6.25/50 Tablet should be given to breastfeeding mothers only if clinically needed.
Driving
Unsafe
CTD-M 6.25/50 Tablet may cause dizziness. So, refrain from activities that require you to stay alert such as driving and operating heavy machinery.
Liver
Caution
CTD-M 6.25/50 Tablet should be used with caution in patients with severe liver diseases. Dose adjustments may be necessary.
Kidney
Caution
CTD-M 6.25/50 Tablet should be used with caution in patients with severe kidney diseases. Regular monitoring of serum electrolyte levels and dose adjustments are necessary.
Children
Unsafe
CTD-M 6.25/50 Tablet is not recommended for use in children.
Heart
Caution
CTD-M 6.25/50 Tablet should be taken with caution in patients with heart problems. Please inform your doctor if you have any pre-existing heart problems before using CTD-M 6.25/50 Tablet .
Geriatrics
Caution
CTD-M 6.25/50 Tablet should be given with caution in elderly patients due to an increased risk of side effects. Seek medical advice before using CTD-M 6.25/50 Tablet .
FAQs
CTD-M 6.25/50 Tablet is used to treat hypertension (high blood pressure). It can also reduce oedema (fluid retention) in hypertensive patients.
CTD-M 6.25/50 Tablet is a combination of two medicines: Metoprolol and Chlorthalidone. It acts by blocking the action of epinephrine hormone that can increase heart rate and blood pressure and also helps to expel excess fluid out of the body reducing edema or swelling.
CTD-M 6.25/50 Tablet may cause orthostatic hypotension. So, stand up slowly while rising from a lying or sitting position. Also, it causes photosensitivity, so avoid prolonged exposure to sunlight and use a sunscreen of high SPF while going outdoors.
It is advised to limit intake of bananas and other foods rich in potassium such as tomato, avocado, and papaya to avoid the risk of side-effects.
Hypertension is a chronic condition, so CTD-M 6.25/50 Tablet is prescribed for several weeks to months to control the blood pressure and also to reduce complications associated with it.
CTD-M 6.25/50 Tablet may cause side-effects such as anorexia (loss of appetite), nausea and vomiting, diarrhea, constipation, jaundice (yellowing of the whites of eyes and skin), dizziness, headache, visual abnormality, photosensitivity (sensitivity to light), orthostatic hypotension (dizziness on standing due to low blood pressure), weakness, restlessness, bradycardia (slow heart rate), and stomach pain. It may also alter electrolyte levels. If any of these side-effects persist, consult your doctor immediately.
Country of origin
Manufacturer/Marketer address
Disclaimer
Author Details
We provide you with authentic, trustworthy and relevant information
Recommended for a 30-day course: 3 Strips